Athena Review Vol. 5, no. 1
Records of Life: Fossils as Original Sources
15. Sauropsid Reptiles
Amniotes vs Anamniotes
As currently understood, the first reptiles evolved from reptiliomorph amphibians during the Paleozoic era about 335-320 million years ago (mya), in the Late Mississippian or Early Pennsylv
 anian
period. An early, and definitive reptile innovation was the amniotic
membrane, which surrounds and protects the developing embryo in all
reptiles, birds, and mammals (fig.1). This provided a major advantage
for reptiles over amphibians, in that the reptiles could move away from
water into inland and upland environments, and exploit more food
resources.
anian
period. An early, and definitive reptile innovation was the amniotic
membrane, which surrounds and protects the developing embryo in all
reptiles, birds, and mammals (fig.1). This provided a major advantage
for reptiles over amphibians, in that the reptiles could move away from
water into inland and upland environments, and exploit more food
resources.The term Amniota was introduced by Ernst Haeckel in the 1860s and 70s as a superclass for all reptiles, birds and mammals. Amphibians, tetrapods, and fish, on the other hand, who lack this trait, are classified as Anamniotes. The first eggs provided with an amniotic membrane were probably small, lacked a shell or outer leather membrane, and were laid by small adult females in a transitional environment such as moist leaf litter, that stayed humid and protected both the adult and the egg from desiccation.
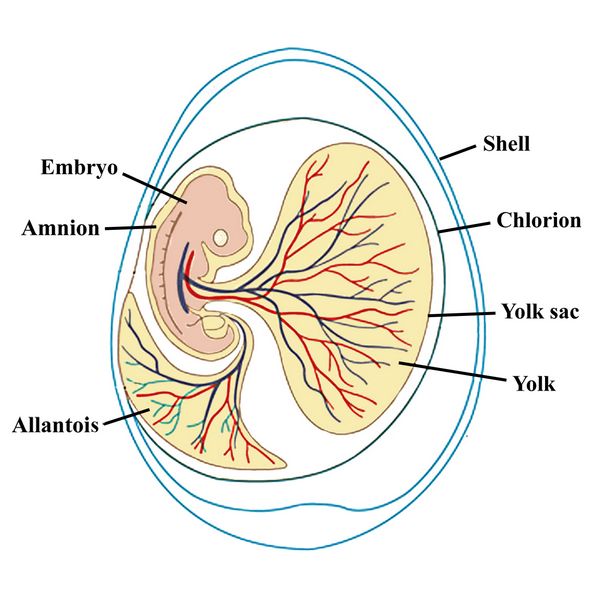
Fig.1: Diagram of the amniotic egg of a reptile.
The reptile egg (fig.1), besides having the amnion or protective membrane over the embryo, has two attached sacs, one called the allantois which exchanges fluids with the embryo, and the other the yolk sac which contains food. Around these is an inner wall called a chlorion, with all being encompassed by a shell.
"Lizard faces" and anapsids, diapsids, eurapsids, and synapsids
Primitive reptiles were split in the late 19th and early 20th century into two major branches, sauropsids and therapsids. The first branch, the Sauropsids ("lizard faces") include the forerunners of most
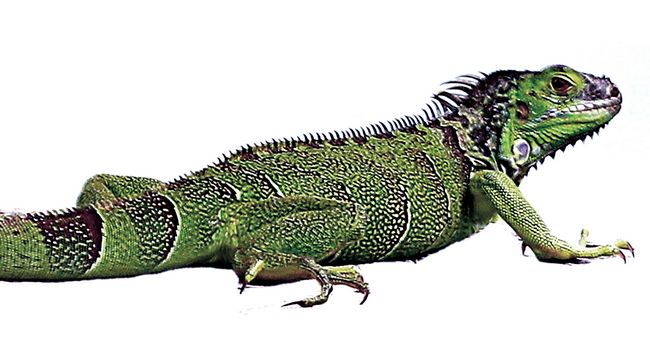 reptiles, from dinosaurs to crocodiles, turtles, and iguanas (fig.2).
The term Sauropsid for ancestral reptiles was coined by Thomas Henry
Huxley in 1863, who grouped the vertebrate classes informally into
mammals, sauroids, and ichthyoids (the latter containing the amphibians
and fish). He subsequently proposed the names of Sauropsida and
Ichthyopsida for reptiles (amniotes) vs. amphibians and fish
(anamniotes).
reptiles, from dinosaurs to crocodiles, turtles, and iguanas (fig.2).
The term Sauropsid for ancestral reptiles was coined by Thomas Henry
Huxley in 1863, who grouped the vertebrate classes informally into
mammals, sauroids, and ichthyoids (the latter containing the amphibians
and fish). He subsequently proposed the names of Sauropsida and
Ichthyopsida for reptiles (amniotes) vs. amphibians and fish
(anamniotes).Fig.2: The iguana, a sauropsid reptile.
The term Sauropsid was reused later by E.S. Goodrich in 1916, in contrast to Therapsids or "beast faces," the latter referring to Late Permian mammal-like reptiles from South Africa which by then were abundantly known. Sauropsids included lizards, birds and their relatives, as distinguished from mammals and their extinct ancestors, grouped as Therapsids. According to this scheme, both Sauropsids and Therapsids evolved from an earlier stem group, the Protosauria ("first lizards"), which included some Paleozoic amphibians as well as early reptiles predating the sauropsid/therapsid split.
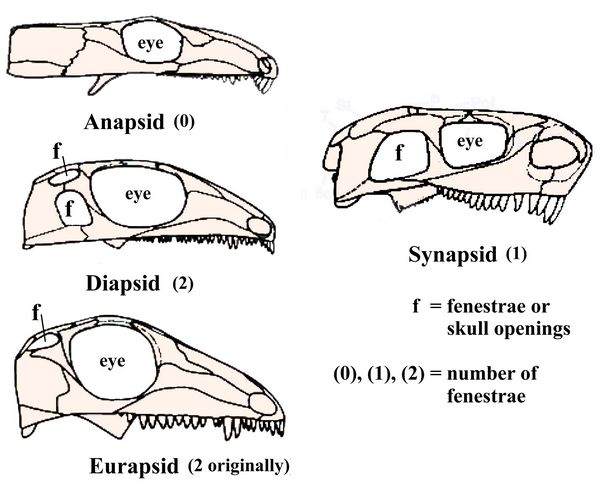
Romer (1956) later subdivided the sauropsids into three basic groups, defined by the number of openings or fenestrae (Latin for "windows") in the side of the skull, used for jaw muscle attachment. This anatomical criteria for all reptiles remains in widespread use (Benton 2005). The anapsids, with no fenestrae, include many basal reptiles or eureptiles, some lizards, and perhaps also the turtles (molecular studies, however, indicate the placement of turtles within diapsids). The diapsids, with two fenestrae, produced the dinosaurs, and more recently, the birds. Huxley (1876) had correctly inferred that birds, who are diapsids, evolved from lizards including dinosaurs, which are also diapsids. An offshoot group of the diapsids, called the eurapsids, with two fenestrae fused into one, produced the large marine reptiles called ichthyosaurs, including Pleisiosaurs and Mosasaurs who were common during the Cretaceous.
Fig.3: Skull openings or fenestrae (after Benton 2005).
Since the late 1960s advent of the cladistic approach to classification and its strict focus on phylogeny or evolutionary links, the term Reptilia has fallen out of favor as too imprecise, with the term Sauropsida used in its place to include a monophyletic group containing reptiles and birds. It seems very likely, however, that common usage will long retain traditional distinctions between Reptiles, Amphibians, Fish, and Mammals (all shown as Classes in the Linnean scheme).
Synapsids and "beast faces"
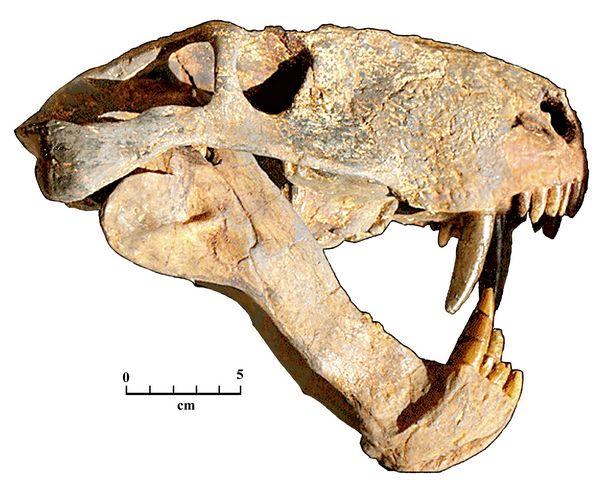
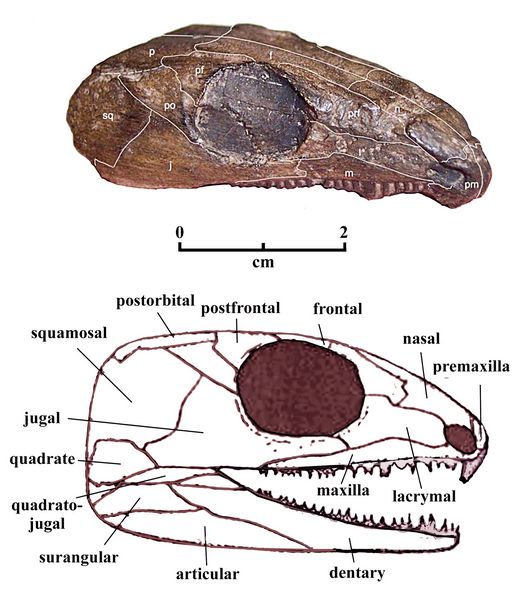
The other major branch of reptiles, the Synapsids (fig.3), have a single large fenestra on each side of the skull, located behind the eyes. The synapsids developed an aorta or arch on the left side only, and strengthened the skull by moving the quadrate bone up and back, eliminating the otic notch. This modification of the jaw (effectively, eliminating the location of the reptilian ear drum, in the otic notch) proved essential in the later development of more complex anatomy related to mammalian hearing (Hunt 1994-7).
 Synapsid
development led to a larger brain, more developed hearing,
heterodont teeth, and homeothermy or regulated body temperature.
The group of synapsids known as the Therapsids ("beast faces"),
equivalent to the term "mammal-like
reptiles," (fig.4) are considered to be the ancestors of mammals, by
way of the
Cynodonts, mammal-like synapsids in the Early and Middle
Triassic. Evidence on transitional fossils bears out the
development of these late, advanced synapsids into early mammals by the
Late Triassic and Early Jurassic. Synapsids and their descendants are
covered in later sections.
Synapsid
development led to a larger brain, more developed hearing,
heterodont teeth, and homeothermy or regulated body temperature.
The group of synapsids known as the Therapsids ("beast faces"),
equivalent to the term "mammal-like
reptiles," (fig.4) are considered to be the ancestors of mammals, by
way of the
Cynodonts, mammal-like synapsids in the Early and Middle
Triassic. Evidence on transitional fossils bears out the
development of these late, advanced synapsids into early mammals by the
Late Triassic and Early Jurassic. Synapsids and their descendants are
covered in later sections.Fig.4: Skull of Aelurognathus tigrinis, a synapsid carnivore from the Late Permian of South Africa; an example of a therapsid or mammal-like reptile, dated 261-254 mya (Mus. fur Naturkunde, Berlin).
Primitive Anapsid Reptiles
During the Middle Carboniferous (Late Mississippian to early Pennsylvanian), according to Benton (2005), the amniote radiation gave rise to three primary clades or branches: the Eureptiles ("true reptiles"), the Anapsids, and the Synapsids. Here Eureptiles are portrayed as a "sister group" of the anapsids, both being subgroups of Sauropsida, the most basal reptilian group, from which Synapsids also derived.
The Eureptiles were terrestrial forms who were still partly lizard-like, characterized by very primitive features, showing that they had only just diverged from their reptiliomorph ancestors (Palaios 2014b). They had rounded, amphibian-like skulls, with most taxa aretaining from fish and tetrapods an opening in the frontal bone for the pineal gland (a “third eye”). They also had amphibian-like shoulders and hips (small, relatively weak pectoral and and pelvic girdles), and limbs (i.e., fused lower leg bones). The rest of their skeleton was mainly reptilian, with spool-shaped vertebral centra.
Romer (1956) defined the order Cotylosaura (“socket-lizards”) as a diverse assemblage of primitive anapsid reptiles containing, among other groups, the Captorhimomorphs, Diadectics, and Pareisours. Of these, only the captorhinomorphs are recognized as being related to more modern reptilians (Heaton 1979). Since they have been defined as being at the base of reptilian phylogeny, a number of detailed descriptions of their anatomy have been made by Carroll (1970), Carroll and Baird (1972), Clark and Carroll (1973), Heaton (1979), and others, who probably would be classified as evolutionary systematists (see section on Classification).
Eureptiles
Casineria kiddi, the type species of Eureptiles, was discovered by an amateur paleontologist in 1992 on the shore of Cheese Bay, Scotland, from formations datings from the Visean phase of the Early Carboniferous or Mississippean (about 335 mya). Casineria is probably the earliest fossil taxa yet classified as an amniote (a basal or proto-reptile). The Archosauromorpha clade which it is assigned to represents the earliest amniotes (Paton, Smithson and Clack 1999).
Casineria was a small insectivore with five fingers, and with claws on each hand, representing the earliest evidence of claws. The development of claws is linked to the formation of keratinous scales in reptiles; thus Casineria very likely had scaly, reptilian skin. The skull of Casineria had no otic notch, as is also the case with another earl
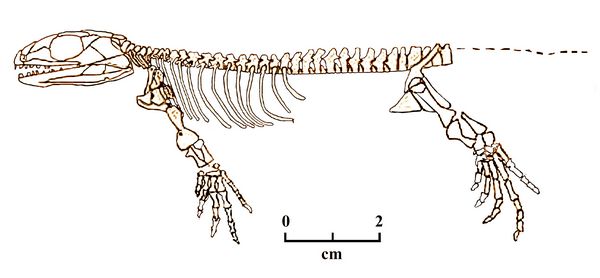 y Eureptile, Brouffia (thus, it probably had no tympanic
membrane or eardrum, and consequently had poor hearing of high
frequency sounds). Casineria had a mixture of anatomical features, some
from the amphibian reptiliomorph groups such as Seymouriamorpha and
Diadectomorpha, but others linking it with early reptiles, such as
lighter leg bones, unfused ankles, and toes terminating in claws.
y Eureptile, Brouffia (thus, it probably had no tympanic
membrane or eardrum, and consequently had poor hearing of high
frequency sounds). Casineria had a mixture of anatomical features, some
from the amphibian reptiliomorph groups such as Seymouriamorpha and
Diadectomorpha, but others linking it with early reptiles, such as
lighter leg bones, unfused ankles, and toes terminating in claws. Fig.5 Casineria kiddi
All this would have enabled the animal to walk more efficiently, and indicates a primarily terrestrial lifestyle. The small size of Casineria is also seen as relevant to a speeded-up evolutionary path, which has probably occurred often in vertebrate branches. Smaller animals have relatively short lifespans, but generally mature faster and thus reproduce more often, which likely had evolutionary significance in the case of Casineria (cf. Carroll 1970)
Cephalerpeton is a small Eureptile found at the Mazon Creek site in Illinois, in the Francis Creek Shale Member of the Carbondale Formation, dating from the late Westphalian stage of the Pennsylvanian ( 306 mya). The type species, Cephalerpeton ventriarmatum, was first named by R. L. Moodie in 1912 .
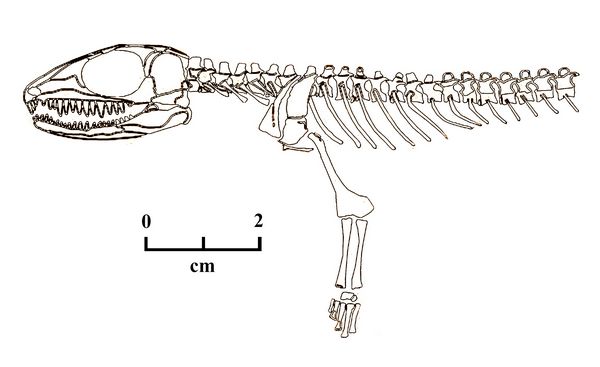
C. ventriamatum, measuring 10 cm in length, has many primitive traits showing similarities with the earlier Reptilioform amphibian, Gephyrostegus. The skull of Cephalerpeton was relatively large with a large orbit, traits typically associated with juveniles. The quadrate was aligned vertically, and the otic notch was greatly reduced, both traits directly relevant to the development of the reptilian middle ear in this taxa.
Fig.6: Cephalerpeton
Specific adaptations related to diet are shown in the jaw and dentition. Its upper (maxillary) teeth were very large, with the mandible concave on its upper (dorsal) surface, in order to accommodate the upper teeth. The maxilla was slightly raised to just above the lower rim of the orbit. the premaxilla was bent downwaad, with the longest premaxillary teeth located toward the center. The palate was relatively shorter. The pterygoid bone in the skull also had a single transverse row of teeth.
More reptilian, land-based traits are seen in the post-cranial skeleton. The cervical vertebrae were elongated, and numbered two more than in Gephyrostegus. The scapula and coracoid were unfused and as tall as the neural spines. The humerus was longer, more slender and hourglass-shaped. The radius and ulna were likewise more slender and relatively longer than in the earlier reptilomorph. Regarding the hands, while only the metacarpal or wrist bones are preserved, they appear to be assymmetrical as in Gephrostegus, with the fourth metacarpal the longest.
Anapsida
Captorhinidae
Captorhinidae is a family of Anapsids dating from the Late Carboniferous (Pennsylvanian) and Early Permian. It was first defined as a family by Case (1911), based on examples of Captorhinus aguti found by Cope (1882) from the Arroyo Formation of the Leonardian phase of the Early Permian (280-270 mya) in northern Texas. The name, Captorhini or "capture-nose", refers to a view that the hook-like premaxilla was used to capture prey (Cope 1882; Palaois 2014b). The family was further defined in numerous studies, including those of Romer (1933, 1956), Fox and Bowman (1957), Clark and Caroll (1973), Hatton (1979), and Kissel et al. (2002).
Captorhinids inhabited an area of Pangea called the Wichita uplift, now part of the Permian Basin in Oklahoma and north Texas. They have broad, robust skulls that are generally triangular or hea
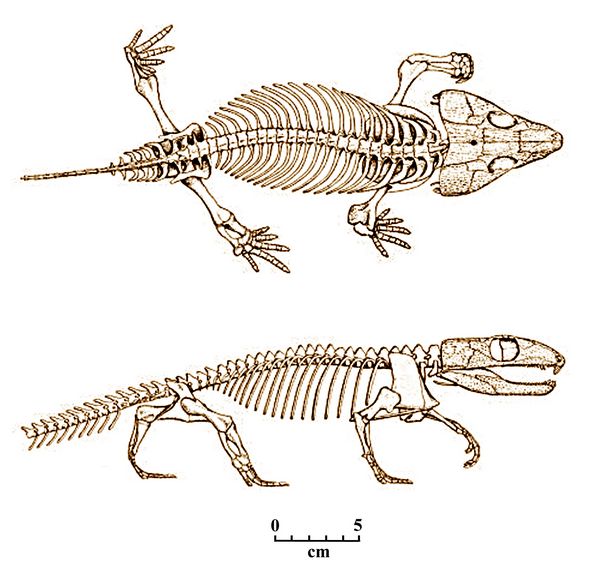 rt- shaped when seen
from above (i.e., in dorsal view), caused by a swollen cheek
region. The premaxillae are characteristically hooked or downturned.
Early, smaller forms including Protocaptorhinus and Romeria possessed
single rows of teeth, while larger, more derived forms such as
Captorhinus aguti have two or three rows of teeth on both the maxillary
and dentary bones. The teeth are short, with chisel-like tips.
rt- shaped when seen
from above (i.e., in dorsal view), caused by a swollen cheek
region. The premaxillae are characteristically hooked or downturned.
Early, smaller forms including Protocaptorhinus and Romeria possessed
single rows of teeth, while larger, more derived forms such as
Captorhinus aguti have two or three rows of teeth on both the maxillary
and dentary bones. The teeth are short, with chisel-like tips.Captorhinus aguti , the type genus for the Captohinidae family, was first found by Cope (1882) in the Clear Fork group of the Permian Basin of northern Texas (280-270 mya). It was a small herbivorous anapsid with a body length about of 40 cm, and a skull length of 5.5 - 8.0 cm. C. aguti is well represented in the Permian basins of Oklahoma (Garber Formation, Sumner Group), and Texas (Arroyo Formation).
Fig.7: Captorhinus laticeps
Captorhinus laticeps ("broad headed Captorhinus") was first discovered in north Texas as a poorly preserved sample by Cope (1882), who named it Ectocynadon. It was later renamed Patriatus laticeps by Williston (1909). After a number of subequent name permutations, on the basis of well-preserved samples from Oklahoma, it was renamed Eocaptorhinus laticeps ("dawn Captorhinus") by Heaton (1979, p.11). Eocaptorhinus, in Heaton;s words (1979, p.5) showed "an astonishing correlation of similar anatomical characters with Sphenadon and primitive iguana lizards, leading to the use of these modern forms as the major source of information." Liike the more primitive forms, Romeria and Protocaptorhinus, C. laticeps has only a single rows of teeth in its dentary and maxilla. The skull of C. laticeps, from 6.5-8.0 cm in length, is larger and wider than those of either Romeria or Proocaptorhinus. Like them, it also had a pineal foramen (third eye) in the top of the skull, a primitive trait.
Captorhinus magnus , the most recently discovered species in the captorhinidae family, and by far the largest, was found in Permian levels at Fort Sill, Oklahoma (Kissel, Dilkes, and Reisz 2002). Some collections at the Ft. Sill site in Oklahoma showed that specimens of C. magnus are more abundant in the lower parts of the section, whereas C. aguti is more abundant in the higher part. All of this would seem to fit into the evolutionary, stratigraphically-based view of Heaton (1979) that C. magnus may be earlier and more primitive than C. aguti. .
Romeria: Two species, Romeria prima and Romeria texana both date from the Asselian stage of the Cisuralian epoch of the Early Permian (299-294 mya). R. texana was found by Llewellyn Ivor Price (1937) at the Cottonwood Creek site, in the Archer City Formation in northern Texas. R. prima, from the same location, was found by Clark and Carroll in 1973 (Heaton 1979) Both species are represented by skulls .
Fig.8: Romeria prima
Romeria has a single set of teeth in its upper and lawer jaws (maxilla and dentary), which Heaton (1979) regarded as a primitive trait, also shared by Protocaptorhinus, Captorhinus laticeps, and Captorhinus major. This contrasts to the dentition of Captorhinus aguti, which is found in later stratigraphic levels, and has two or three rows of teeth in both the maxilla and dentary.
.
.
.
References:
Benton, M., 2005, pp.104-5
Brough, M.C. and J. Brough, 1967. The Genus Gephyrostegus. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 252 (776): pp. 147–165.
Carroll R.L. 1970. The Ancestry of Reptiles. Philosophical Transactions of the Royal Society B 257: pp. 267–308.
Carroll R.L. 1988
Carroll R.L. and Baird D. 1972. Carboniferous Stem-Reptiles of the Family Romeriidae. Bulletin of the Museum of Comparative Zoology 143(5): pp. 321-363.
Case, E.C. 1911: A revision of the Cotylosauria of North America. Carnegie Institution of Washington Publication vol. 145, Washington, D.C., 122 pp.
Cope, E. D. 1882 Third contribution to the history of the Vertebrata of the Permian formation of Texas. Proceedings of the American Philosophical Society 20: pp. 447-461.
Gauthier J.A. 1986. Saurischian monophyly and the origin of birds, . In K. Padian (ed.) The Origin of Birds and the Evolution of Flight. Memoirs of the California Academy of Science 8, Berkeley, California, pp. 1-55.
Gauthier, A., A.G. Kluge, and T. Rowe 1988 The early evolution of the Amniota. in Michael J. Benton (ed.): The Phylogeny and Classification of the Tetrapods, Volume 1: Amphibians, Reptiles, Birds. Syst. Ass. Spec. Vol. 35A, Clarendon Press, Oxford, pp. 108-155.
Goodrich, E.S. 1916 . "On the classification of the Reptilia". Proceedings of the Royal Society of London 89B: pp. 261–276.
Gregory J. T. 1948. The structure of Cephalerpeton and affinities of the Microsauria. American Journal of Science, 246: pp. 550-568
Heaton, M.J. 1979. Cranial Anatomy of Primitive Captorhinid Reptiles from the Late Pennsylvanian and Early Permian, Oklahoma and Texas. Oklahoma Geological Survey, Bulletin vol. 127. The University of Oklahoma, Norman, 83 p.
Heaton, M.J. and R.R. Reisz. A skeletal reconstruction of the early Permian captorhinid reptile Eocaptorhinus laticeps (Williston). Journal of Paleontology 54: 136-143.
Hunt, K. 1994-1997
Haeckel, E. 1866 Generelle Morphologie der Organismen : allgemeine Grundzüge der organischen Formen-Wissenschaft, mechanisch begründet durch die von C. Darwin reformirte Decendenz-Theorie. ) Berlin
Huxley, T.H. 1863. The Structure and Classification of the Mammalia. Hunterian lectures, presented in Medical Times and Gazette.
Huxley, T.H. 1876. Lectures on Evolution. New York Tribune. Extra. no 36. In Collected Essays IV: pp 46-138
Kissel, R.A., D.W. Dilkes, and R.R. Reisz, 2002. Captorhinus magnus, a new captorhinid (Amniota: Eureptilia) from the Lower Permian of Oklahoma, with new evidence on the homology of the astragalus. Canadian Journal of Earth Science, 39 (9), pp. 1363-1372
Laurin, M.; and R. Reisz, 1995 . "A reevaluation of early amniote phylogeny". Zoological Journal of the Linnean Society 113 (2):pp. 165–223.
Laurin, M. and J.A. Gauthier, J.A. 1996 . "Amniota. Mammals, reptiles (turtles, lizards, Sphenodon, crocodiles, birds) and their extinct relatives." Version 1
May, W. J. and R. L. Cifelli 1998. "Baeotherates fortsillensis, A New Captorhinid Reptile from the Fort Sill Fissures, Lower Permian of Oklahoma". Oklahoma Geology Notes 58: pp. 28–33
Moodie, R.L. 1912. "The Pennsylvanic Amphibia of the Mazon Creek, Illinois, Shales". Kansas University Science Bulletin 6 (2): pp. 232–259.
Muller, J. and Reisz, R.R. 2006. . "The phylogeny of early eureptiles: Comparing parsimony and Bayesian approaches in the investigation of a basal fossil clade." Systematic Biology, 55(3): pp. 503-511.
Nor-Eddine, J. and J-M Dutuit 1996 . "Permian captorhinid reptiles from the Argana formation, Morocco". Palaeontology 39 (4): pp. 907–918
Palaios.org 2014a, "Romeridae".
Palaios.org 2014b, "Captorhinidae".
The Paleobiology Database: "Moradisaurinae".
Paton, R. L., T. R. Smithson and J. A. Clack, "An amniote-like skeleton from the Early Carboniferous of Scotland", Nature 398, pp. 508-513
Pawly, K. 2006. "Walking with early tetrapods: evolution of the postcranial skeleton and the phylogenetic affinities of the Temnospondyli (Vertebrata: Tetrapoda)." Chapter 6: in The postcranial skeleton of temnospondyls (Tetrapoda: temnospondyli) PhD Thesis. La Trobe University, Melbourne.
Price, L. I. 1937. "Two new cotylosaurs from the Permian of Texas". Proceedings of the New England Zoölogical Club 11: pp. 97–102.
Basal reptiles " Reptileevolution.com
Romer, A.S. 1952.. "Late Pennsylvanian and Early Permian Vertebrates of the Pittsburgh-West Virginia Region". Annals of Carnegie Museum 33: pp. 47–113.
Romer, A.S. 1966. Vertebrate Paleontology. University of Chicago Press., 3rd ed. (orig. 1933).
Watson, D.M.S. 1957. "On Millerosaurus and the early history of the sauropsid reptiles". Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 240 (673), pp. 325–400.
Wikiipedia 2014a; "Sauropsids and Anapsids".
Williston, S.W. 1909. New or Little Known Permian Vertebrates. Pariotichus. Biological Bulletin 17(3), pp. 241-255.
Williston, S.W. 1911
Glossary