Athena Review Vol. 5, no. 1
Records of Life: Fossils as Original Sources
6. Precambrian Life: Archaean and Proterozoic
The Precambrian period lasted from the initial formation of the Earth (estimated at about 4.5 billion years ago) to the beginning of the Cambrian period (542 million years ago), when complex marine animals began to flourish. For most of the 4 billion years of the Precambrian, the only living inhabitants of Earth were one-celled microbes resembling bacteria. Records of life for this incredibly long period remained unknown until the mid-20th century.
The length of Precambrian time.
The Precambrian is eight times as long as the remaining seventeen geological periods put together. If a graph scale nine inches long represents the 4.5 billion year age of the earth (fig.1), the first eight inches are Precambrian time, and only the last inch (since about 542 million years ago) represents the Cambrian and later periods.
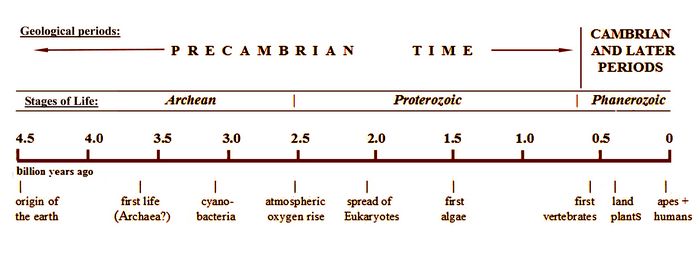
Fig.1: Time chart showing the relative length of Precambrian time.
This covers the origins of vertebrates from fish through humans, and of all land plants. Human ancestors first appeared a little over 5 million years ago, in the last one-hundredth of an inch on this scale.The Precambrian is split up into three successive “eons” which reflect the development of the Earth and its early life.
The earliest, informally called the Hadean by geologists, dates from the initial formation of Earth, estimated at about 4.5 billion years ago, until about 4 billion years ago. The Hadean has no known surviving rock formations, and thus no surviving fossil evidence of the earliest life on Earth. During this initial time frame, Earth may have been inhospitable to any life forms. Some paleontologists including Woese (1987), however, have speculated that some archaeans who inhabit extreme environments may have existed then.
The first stages of early life are called, successively, the Archean and the Proterozoic eons. The Archean, lasting from about 3900-2600 mya, represents the earliest rock formations preserved in continental bedrock masses, called cratons. The oldest, dated at about 3.5 billion years ago, are found in Australia, Canada, and South Africa. These contain the earliest known life forms in the fossil record, which consist of mat-like layers of fossil imprints called stromatolites (fig.2). These represent traces of large colonies of microbes which occupied shallow coastal water settings, of the type that today might contain coral reefs.
 One
such site is a chert formation in the Pilbara Craton, located in
northwest Australia, This has been identified as one of the
earliest well preserved crustal formations, along with the Kaapvaal
Craton in South Africa. Both locations may have once been part of an
early land mass called the Vaalbara Supercontinent. In the Strelley
Pool Chert formation in the Pilbara Craton, sections of layered
stromatalites are preserved, dating from the Paleoarchaean stage,
between 3.6-3.2 billion years ago (fig.2). Stromatolites over 3 billion
years old have also been found in South Africa (Taylor et al. 2009).
Assuming their interpretation is correct, these stromatolites
represent the earliest known life on Earth.
One
such site is a chert formation in the Pilbara Craton, located in
northwest Australia, This has been identified as one of the
earliest well preserved crustal formations, along with the Kaapvaal
Craton in South Africa. Both locations may have once been part of an
early land mass called the Vaalbara Supercontinent. In the Strelley
Pool Chert formation in the Pilbara Craton, sections of layered
stromatalites are preserved, dating from the Paleoarchaean stage,
between 3.6-3.2 billion years ago (fig.2). Stromatolites over 3 billion
years old have also been found in South Africa (Taylor et al. 2009).
Assuming their interpretation is correct, these stromatolites
represent the earliest known life on Earth.Fig.2: Fragment from a layered stromatolite feature in the Strelley Pool Chert in Pilbara Craton in Australia, dated at over 3 billion years ago.
Prokaryotes and Eukaryotes.
Based on analogy with more recent stromatolites, which are found in many places around the world, the microbes represented in the stromatalite fossils from the Archean time frame were all simple, one-celled organisms similar to bacteria, called prokaryotes or "proto-cells". Prokaryotes are defined on the basis of having a relatively simple, primitive cell with no distinct nucleus, in contrast with eukaryotes ("true cells") which have distinct nuclei and organelles. Most prokaryotes merely have an outer membrane or cell wall, while the DNA molecules which encode the protein production of the prokaryote simply float around within its cell wall. They thus may possibly combine with any other DNA that may be encountered; reproduction was not yet controlled by having the cell's DNA contained in a nucleus. Some prokaryotes known as cyanobacteria, however, as discussed below, do contain organelle-like elements called carboxysomes which contain the enzyme called Rubisco, essential to photosynthesis (fig.3).
The first appearance of eukaryotes is estimated at about 2.5 billion years ago, when the oxygen content in the atmosphere had risen due to photosynthesis by early cyanobacteria (see below). This commences the Proterozoic eon of the Precambrian, which lasts from about 2600 to 750 mya (Margulis and Schwartz 1988).
Cyanobacteria and photosynthesisls
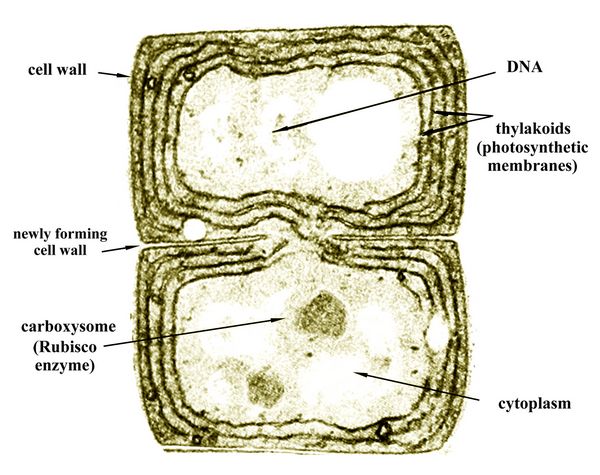 The
early prokaryotes who left their imprints in the mat-like stromatalite
fossils were mainly cyanobacteria ("blue-green bacteria"). They used
photosynthesis, and thus were autotrophs ("self-feeders"), in contrast
to heterotrophs who eat other organisms or organic compounds such as
methane. Like many algae filling the Earth's waters today, the
Precambrian cyanobacteria lived in colonies. Their DNA floated around
in the cytoplasm inside the thylakoids, and they reproduced by cell
division or fission (fig.3).
The
early prokaryotes who left their imprints in the mat-like stromatalite
fossils were mainly cyanobacteria ("blue-green bacteria"). They used
photosynthesis, and thus were autotrophs ("self-feeders"), in contrast
to heterotrophs who eat other organisms or organic compounds such as
methane. Like many algae filling the Earth's waters today, the
Precambrian cyanobacteria lived in colonies. Their DNA floated around
in the cytoplasm inside the thylakoids, and they reproduced by cell
division or fission (fig.3). Fig.3: Two cells of the freshwater cyanobacteria Anabaena (after Margulis and Schwartz 1988, p.49)
To produce the glucose or carbohydrates used for food via photosyntheisis, their cells contained folded layers of tissue called thylakoids (fig.3), each containing chlorophyll pigments for catching photons from sunlight. The photons were used as hydrogen ions to convert phosphate molecules into the energy storing molecule Adenine Triphosphate (ATP). This then was used to create glucose or starch from carbon dioxide. Cyanobacteria cells also contain microcompartments called carboxysomes (fig.3), filled with molecules of the enzyme Rubisco, which serves as a catalyst for photosynthesis.
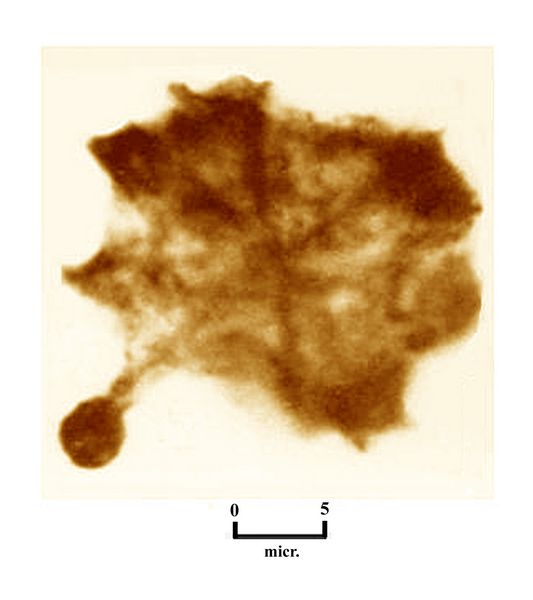 The
first well-preserved fossils of individual prokaryotes were reported in
the mid-20th century, with the discovery by Tyler and Barghoorn (1954)
of an assortment of fossil prokaryotes dating from about 1.9 billion
years ago. These were found in the Gunflint chert Formation of Ontario,
Canada near Lake Superior (see also Barghoorn and Tyler 1965, and
Awramik and Barghoorn 1977). Some of the microscopic fossils preserved
in the Gunflint chert were cyanobacteria, and other forms seemed
completely new.
The
first well-preserved fossils of individual prokaryotes were reported in
the mid-20th century, with the discovery by Tyler and Barghoorn (1954)
of an assortment of fossil prokaryotes dating from about 1.9 billion
years ago. These were found in the Gunflint chert Formation of Ontario,
Canada near Lake Superior (see also Barghoorn and Tyler 1965, and
Awramik and Barghoorn 1977). Some of the microscopic fossils preserved
in the Gunflint chert were cyanobacteria, and other forms seemed
completely new.Fig.4: Kakabekia umbellata, a prokaryote with an umbrella-like mantle from the Gunflint Chert Formation in Canada, dating from 1.9 billion years ago (photo: after Schopf et al. 1965)
One of the most unusual prokaryotes from Gunflint is Kakabekia umbellata, named for nearby Kakabeka Falls in Ontario, and notable for its umbrella-like crown or mantle (fig.4). As one of a number of Gunflint fossils revealed by electron microscopy (Schopf et al. 1965), Kakabekia had three parts, a bulb-shaped base, from which projected a main stem or stipes, at the end of which was an umbella-like mantle, up to 30 microns in diameter, supported by 6-8 radial spokes. At the time of their original taxa descriptions, Barghoorn and Tyler (1965) could not identify any relationship with other known organisms.
In what seems an amazing coincidence, however, one had already been found. In 1964, when biologist S. M. Siegal of the University of Hawaii was experimenting with ammonia-tolerant organisms, as part of a project to describe possible lifeforms surviving in extreme conditions, he came across a tiny, umbrella-shaped form in soil samples from Wales. When he saw the 1965 Barghoorn and Tyler paper on the Gunflint Chert fossils, he recognized the same form in their Kakabekia umbellata. After more research to confirm the similarities, based on a number of examples studied, Siegal and his colleagues announced the new species Kakabekia barghoorniana (fig.5), as a living fossil related to, and closely resembling the two-billion-year-old Kakabekia umbellata (Siegal et al. 1967).
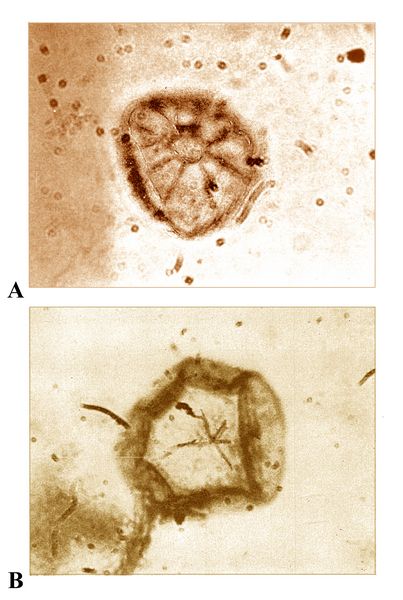 In describing K. barghoorniana
in this, and a subsequent paper (Siegal and Siegal 1970), they also
provided a quantity of useful detail that can probably be extrapolated
back with some reliability to the fossil species. For example, K. barghoorniana
(fig.5) lacks a nucleus, so is definitely a prokaryote. It has to live
in or obtain ammonia, thus derives its energy from ammonia, and has no
storage of lipids or polysaccharides; it is anaerobic, but can tolerate
oxygen. Regarding reproduction and life cycle, spore distribution was
observed, along with differentiation of the umbrella-like mantle into
its "mature" form, followed by disintegration or senescence of this
stage. The stalk and basal bulb of the complete umbellate structure
formed part of the reproductive apparatus. In parallel with the fossil
forms, the live organism also showed a number of variant forms of both
mantle and main stipes, sometimes lacking the latter. The mantle
usually had 6-8 radial spokes or branches, which meet in a ring-shaped
central feature (fig.5A).
In describing K. barghoorniana
in this, and a subsequent paper (Siegal and Siegal 1970), they also
provided a quantity of useful detail that can probably be extrapolated
back with some reliability to the fossil species. For example, K. barghoorniana
(fig.5) lacks a nucleus, so is definitely a prokaryote. It has to live
in or obtain ammonia, thus derives its energy from ammonia, and has no
storage of lipids or polysaccharides; it is anaerobic, but can tolerate
oxygen. Regarding reproduction and life cycle, spore distribution was
observed, along with differentiation of the umbrella-like mantle into
its "mature" form, followed by disintegration or senescence of this
stage. The stalk and basal bulb of the complete umbellate structure
formed part of the reproductive apparatus. In parallel with the fossil
forms, the live organism also showed a number of variant forms of both
mantle and main stipes, sometimes lacking the latter. The mantle
usually had 6-8 radial spokes or branches, which meet in a ring-shaped
central feature (fig.5A).Fig.5: Kakabekia barghoornia, the extant relative of K. umbellata, showing two variants of its mantle or parachute-like form: A) a fully developed radial system with a well-defined central feature; B) an incomplete radial system, with the mantle margin folded over; part of the main stipes or stem is visible below (from Siegal and Siegal 1970, fig.3)
This information provided insight on the possibilities of life forms from the Early Proterozoic, about two billion years ago - and shows the tenacity of life forms from that remote era. Since the original discovery of K. barghoorniana in Wales, the tiny organism has also been found in soil samples from Alaska, Iceland, Nepal, Mexico, Columbia, Equador, and Hawaii. Given this range of environments, K. barghoorniana is definitely cold-tolerant.
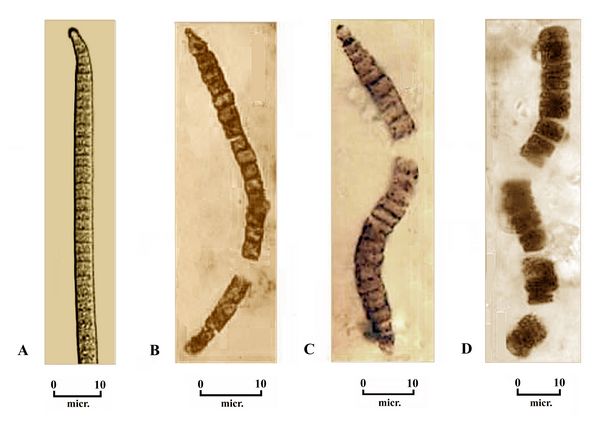 Other
parallels with current species can be seen fossil deposits of
cyanobacteria from Bitter Springs, Australia, in chert and quartzite
deposits dating from the Late Proterozoic, about 850 million years ago
(Schopf 1968). These deposits are rich in fossils of certain forms of
segmented bacteria (fig.6). Some are very close in form to Oscillatoria amena (fig. 6A), an extant genus of filamentous cyanobacterium, named for its oscillating movement in seeking light sources.
Other
parallels with current species can be seen fossil deposits of
cyanobacteria from Bitter Springs, Australia, in chert and quartzite
deposits dating from the Late Proterozoic, about 850 million years ago
(Schopf 1968). These deposits are rich in fossils of certain forms of
segmented bacteria (fig.6). Some are very close in form to Oscillatoria amena (fig. 6A), an extant genus of filamentous cyanobacterium, named for its oscillating movement in seeking light sources. Fig.6: Cyanobacteria fossils from the Bitter Springs chert formation in Australia, 850 mya: A) The extant bacteria, Oscillatoria amena. B) a similar fossil genus, Filiconstrictosus.C) the fossil species Cephalophytarion grande. D. the fossil Oscillatoriopsis sp. (photos after Taylor et al. 2009, and Schopf 1968.)
Endosymbiosis as the origin of eukaryotes and organelles.
According to a widely held theory, the emergence of eukaryotes or one-celled organisms with complex, nucleated cells began when prokaryotes began to function in symbiotic relationships. Thus, one unicelled organism was absorbed by another and remained alive to function as an organelle, in a process called endosymbiosis (Margulis and Schwartz 1988). Endosymbiosis is thought to be crucial for the early evolution of eukaryotic cells, including the origins of both the mitochondrion, an essential energy producing device in all eukaryotes, and the chloroplast, which is essential for photosynthesis.
Organelles: mitochondria for energy production.
Based on the fossil and genetic records, this may have occurred by about 1.8 billion years ago, i.e., a little later than the fossil evidence from the Gunflint chert (Barghoorn and Tyler 1965), where only prokaryotes were found. The earliest instances of endosymbiosis may have involved the absorption of a heterotrophic prokaryote which produced
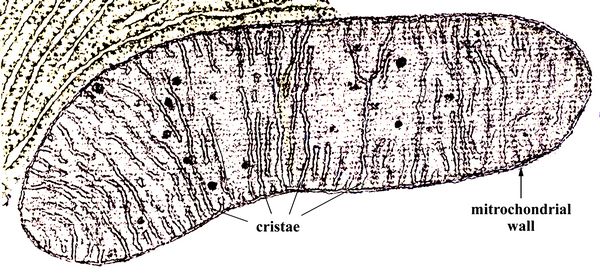 ATP
by another heterotroph. The captured ATP-producing cells became
energy-producing organelles of the host cells, called mitochondria.
ATP
by another heterotroph. The captured ATP-producing cells became
energy-producing organelles of the host cells, called mitochondria. Fig.7: A mitochondrion, imaged by a scanning electron microscope (SEM). The cristae membranes are indicated.
Mitochondria are filled with ATP-producing membranes called cristae (fig.7). The mitochondrion acquired its own double-surrounding membrane, of which the innermost also forms the bounded enclosures walled with cristae. The mitochondrion also retains its own DNA. The original DNA of the host cell, meanwhile, became enclosed in a cell nucleus. Part of the mitochrondial DNA migrated to the main cell nucleus, but part of it remained with the mitrochondria organelle, which could then reproduce independently by cell division within the host cell.
Mitochondrial DNA provides one of the most important methods for comparing one organism with another, and determining when they may have split off from common ancestors. All eukaroyotes, whether plants, animals, fungi, or protcists like one-celled algae, have mitochondria, which in bisexual animals descends only from the maternal side Mitochondria are thought to have arisen from a bacterium that was already endosymbiotic (Woese 1987). Phylogenetic analyses based on the DNA-encoded proteins in mitochondrial DNA have established that this bacterium was a member of the group called alpha-proteobacteria (Lang et al. 1999; Espeleta and Embley 2012).
Such symbiotic mergers, which probably happened many times in the Precambrian (and which still occur), resulted in a variety of eukaryotes, one celled organisms with complex nucleated cells (Margulis and Schwartz 1988). The first fossil evidence of eukaryotes marks the start of the Proterozoic eon, which lasts until the end of the Precambrian.
Organelles: chloroplasts for photosynthesis.
The second important example of endosymbiotic mergers involved transformation of cyanobacteria into chloroplasts
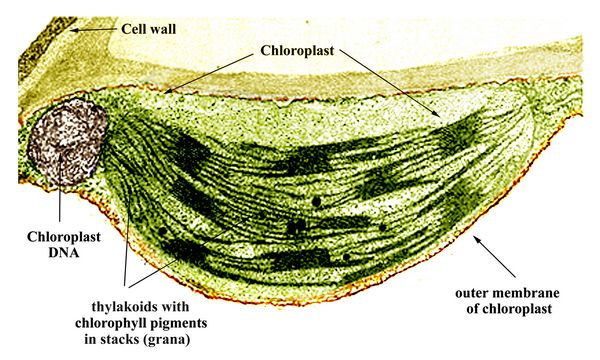 (fig.8),
which produce photosynthesis in algae and plants. It is generally
accepted that chloroplasts in eukaryotic photosynthetic organisms were
derived through endosymbiosis, after the capture of a cyanobacterium by
an early eukaryote. This primary endosymbiosis resulted in the
ancestor of red algae and green algae. Much later, during the Paleozoic
era, one branch of green algae, the Chlorophyta, became the ancestors
of land plants.
(fig.8),
which produce photosynthesis in algae and plants. It is generally
accepted that chloroplasts in eukaryotic photosynthetic organisms were
derived through endosymbiosis, after the capture of a cyanobacterium by
an early eukaryote. This primary endosymbiosis resulted in the
ancestor of red algae and green algae. Much later, during the Paleozoic
era, one branch of green algae, the Chlorophyta, became the ancestors
of land plants.Fig.8: Chloroplast of a land plant leaf, showing the stacked layers of thylakoids which contain chlorophyll pigments. Each stack of thylakoids is called a granum.
In a scenario thought to have occurred between about 1.8 and 1.5 billion years ago, a cyanobacterium taken in by a eukaryotic host cell evolved into the original chloroplast, an organelle named for its chlorophyll pigments. These pigments, used for capturing sunlight photons in photosynthesis, are contained in layers of thylakoid tissue. Like the mitochondria, the chloroplasts also retained some of their original DNA. The chloroplast, however, has a greatly reduced genome, indicating that most of the original cyanobacterial genes were either lost or transferred into the host nuclear genome. For example, many of the proteins for photosynthesis are now encoded by nuclear genes. Chloroplast DNA nevertheless provides a unique focus of genetic comparison between different autotrophic organisms which perform photosynthesis, including algae, plants, and diatoms.
The resultant eukaryotic organism was a primitive algae, the ancestor of land plants as well as algae in all its marine and freshwater forms of seaweed, diatoms, and pond growths. A widespread example is Chlamydomonas (fig.9), a coccoid (spherical) green algae whose flagella give it mobility, and which is found in both salt and fresh water habitats.
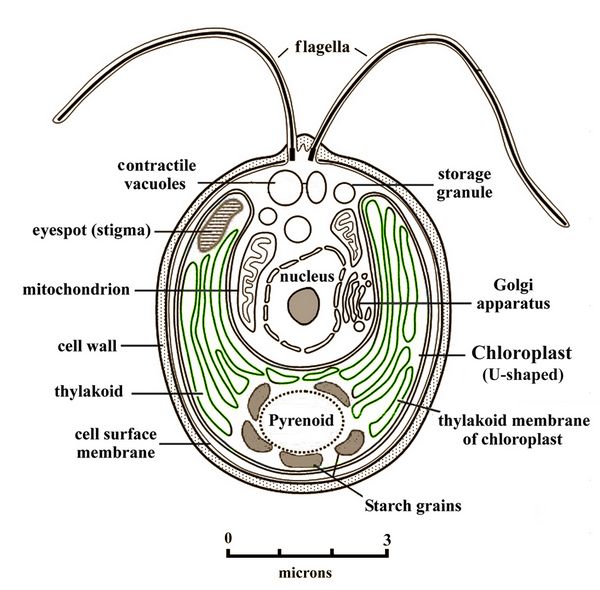 Fig.9:
Cell of Chlamydomonas, a widespread green algae with twin flagella,
showing its chloroplast, mitochrondria, Golgi apparatus, and an
eyespot ennabling light source detection.
Fig.9:
Cell of Chlamydomonas, a widespread green algae with twin flagella,
showing its chloroplast, mitochrondria, Golgi apparatus, and an
eyespot ennabling light source detection.After the origins of red and green algae, secondary symbiotic events occurred, involving the capture of either red or green algae by other eukaryotes. This produced a diversity of secondary strains of algae, including brown algae (represented by kelp and other familiar seaweeds), golden algae (glaucophytes), and yellow-green algae such as Vaucheria litorea, a favorite food of sea slugs (see below). In these algae, the chloroplasts have one or two additional membranes compared with those in either red or green algae, or the land plant descendents of green algae. There is also either partial or complete loss of the nuclear genome of the captured red/green algal cells. Another offshoot from red and green algae are Euglenoids, which selectively perform either photosynthesis or heterotrophy (see below). Given the remote antiquity of both the primary and secondary endosymbioses involved in the evolution of these organisms, there are few, if any, known intermediate cases, making such origins difficult to understand.
Regarding identification of the original cyanobacteria ancestral to chloroplasts, controversy has centered on the origins of the two Chlorophyll pigments a and b, found in both plants and green algae. For a long time, no cyanobacteria were known to have Chlorophyll b, thus raising doubts about the role of cynobacteria in an original endosymbiosis. In 1975, however, the prokaryote genus Prochloron ("before green"), typically found in colonies attached to tunicates, was discovered to contain both a and b Chlorophylls (Lewin 1975). In support of the endosymbiotic theory, Prochloron was for a time considered a candidate for the ancestor of chloroplasts (Woese 1987). More recent genetic studies, however, indicated Prochloron is not on the same line of descent that lead to chloroplast-containing algae and land plants (La Roche et al., 1996). Prochloron is now placed in the group Prochlorophytes along with two other photosynthetic bacterial taxa, Prochlorothrix and Prochlorococcus, the latter perhaps the most widespread of marine phytoplankton. While morphologically resembling cyanobacteria, the prochlorophytes are distinct from them, in that they 1) have stacked thylakoids (i.e., grana-like structures), not found in cyanobacteria; b) use a unique photosynthetic pigment, divinyl-chlorophyll, to absorb light and acquire energy; and c) also lack the red and blue Phycobilin pigments found in cyanobacteria.
Countless types of bacteria currently live in extracellular colonies within host plants and animals, such as the digestive bacteria in all humans, the grass-digesting bacteria in cows’ stomachs, or the cellulose-digesting bacteria within wood termites. Such extracellular colonies are quite distinct from the process of endosymbiosis. Yet the liklihood of endosymbiotic relations having occurred between different kinds of organisms various times throughout the history of
 life
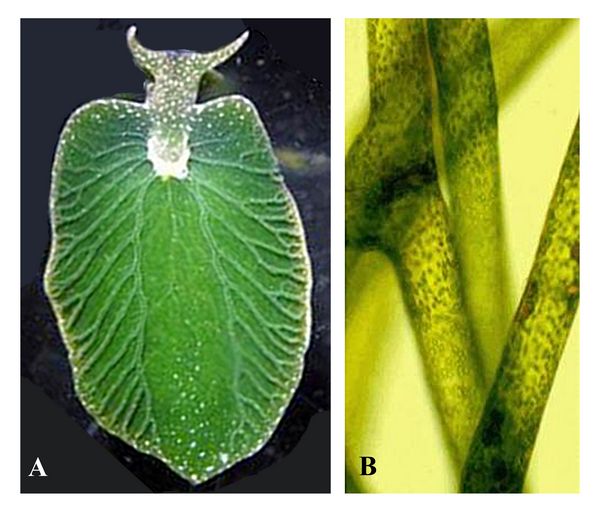
is high. One current example features the “borrowing” of photosynthesis
by the sea slug Elysia chlorotica, a heterotroph which eats the algae
Vaucheria litorea (fig.10). Elysia retains the chloroplasts from
Vaucheria in cells lining its digestive tract. The sea slug has
acquired photosynthesis-supporting genes by horizontal gene transfer
from Vaucheria, and can use the chloroplasts to carry out
photosynthesis for several months (Rumpho et al. 2005).
life
is high. One current example features the “borrowing” of photosynthesis
by the sea slug Elysia chlorotica, a heterotroph which eats the algae
Vaucheria litorea (fig.10). Elysia retains the chloroplasts from
Vaucheria in cells lining its digestive tract. The sea slug has
acquired photosynthesis-supporting genes by horizontal gene transfer
from Vaucheria, and can use the chloroplasts to carry out
photosynthesis for several months (Rumpho et al. 2005).Fig.10: A) Elysia chloritica, a sea slug made green and photosynthetic by ingesting the chloroplasts from the yellow-green algae shown at right, B) Vaucheria litorea.
Chloroplasts do not occur in the cells of either fungi or animals, which are all heterotrophic, but they do occur in some algae descendents called Euglenoids, which can also be heterotrophic in deep sea conditions lacking sunlight. Such chaemera-like organisms crosscut any rigid boundaries between autotrophic plants and heterotrophic animals; many such organisms could also have occurred in Archaean or Proterozoic times.
References:
Awramik, S.M. and E.S. Barghoorn 1977. "The Gunflint Microbiota." Precambrian Research 5, pp. 121-142.
Barghoorn, E.S. and S.A. Tyler 1965. "Microorganisms from the Gunflint chert." Science 147, pp. 563-577.
Benton, M.J. 2005. Vertebrate Paleontology.
Cloud, P. 1989. , pp. 42, and 233-234.
Espeleta, N.R. and T.M. and Embley 2012. "The SAR-11 Group of Alpha-Proteobacteria is not related to the Origin of Mitochondria." Plos One.
Glaessner, M.F. and B. Daly 1959. "The Geology and Late Cambrian Fauna of the Ediacara Fossil Reserve." Records of the South Australian Museum 13, pp. 369-401.
Glaessner, M.F.and M. Wade, 1966. "The late Precambrian fossils from Ediacara, South Australia". Palaeontology 9 (4).
Knoll 2004
Lang, B.F., M.W. Gray, and G. Burger 1999. "Mitochondrial genome evolution and the origin of eukaryotes." Annual Review of Genetics 33, pp. 351–397.
La Roche et al., 1996
Lewin 1975
Li, C-W, J-Y Chen, and T-E Hua 1998: "Precambrian Sponges with Cellular Structures." Science 279, pp. 879 - 882.
Margulis, L. and K.V. Schwartz 1988. Five Kingdoms. An Illustrated Guide to the Phylla of Life on Earth. New York, W.H. Freeman (2nd ed.).
Rumpho-Kennedy, M.E., M. Tyler, F.P. Dastoor, J. Worful, R. Kozlowski, and M. Tyler, M 2006. "Symbio: a look into the life of a solar-powered sea slug." Proceedings of the National Academy of Sciences of the USA
Schopf, J.W. 1968. "Microflora of the Bitter Springs Formation, Late Precambrian, Central Australia." Journal of Paleontology 42(3), pp. 651-688.
Schopf, J.W. 1999. Cradle of Life: The Discovery of Earth's Earliest Fossils. Princeton Univ. Press, Princeton.
Schopf, J.W., E.S. Barghoorn, M.D. Maser, and R.O. Gordon 1965. "Electron Microscopy of Fossil Bacteria Two Billion Years Old." Science 149, pp. 1365-1367.
Siegal, B.Z. and S.M. Siegal 1970. "Biology of the Precambrian genus Kakabekia: New Observations on Living Kakabekia barghoorniana." Proceedings of the National Academy of Sciences of the USA 67(2), pp. 1005-1010.
Siegal, S.M., K. Roberts, H. Nathan, and O. Daly 1967. " Living Relative of the Microfossil Kakabekia." Science 156, pp. 1231-1234.
Spriggs, R.J. 1947. "Early Cambrian (?) Jellyfishes from the Flinders Ranges, South Australia." Transactions of the Royal Society of South Australia 71, pp. 212-224.
Taylor, E.L., T.N. Taylor, and M. Krings 2009. Paleobotany: The Biology and Evolution of Fossil Plants. New York, Academic Press.
Tyler, S.A. and E.S. Barghoorn 1954. "Occurrence of structurally preserved plants in Precambrian rocks of the Canadian Shield." Science 119, pp. 606-608.
Wade, M.J. 1968. "Preservation of soft-bodied animals in Precambrian sandstones at Ediacara in South Australia." Letheia 1, pp. 238-267.
Whately and Lewin 1977
Woese C. R. 1987. "Bacterial evolution." Microbiology Reviews 51, pp. 221–271.
Xiao, S., Y. Zhang, and A.H. Knoll 1998. "Three-Dimensional Preservation of Algae and Animal Embryos in a Neoproterozoic Phosphorite." Nature 391, pp. 553-558.
Xiao, S., X.Yuan, and A.H. Knoll 2000: "Eumetazoan Fossils in Terminal Proterozoic Phosphorites?" Proceedings of the National Academy of Sciences of the United States, 97 (25), pp. 13684-13689.
Glossary